González1, Y. Ellahioui 1, L. Gallego-Yerga, A. Vicente-Blázquez1,2, R. Álvarez1, M. Medarde1, R. Peláez1.
1 Department of Pharmaceutical Sciences, Faculty of Pharmacy, University of Salamanca, Campus Miguel de Unamuno, E-37007 Salamanca, Spain, raquelalvarez@usal.es. 2 Laboratory of Cell Death and Cancer Therapy, Biological Research Center, CSIC, E-28040 Madrid, Spain.
Keywords: tubulin, colchicine site, solubility, antimitotic, apoptosis, amino substituents.
Antimitotic agents that bind to tubulin at colchicine site have serious drawback because of their low aqueous solubility. [1] MPGI strategy is key in order to design new ligands that include polar amino groups that behave as non-polar residues when bound to tubulin. [2] In this work we have design and synthesized compounds in which the classical trimethoxyphenyl moiety of colchicine site antimitotic ligands is replaced by substituted anilines. [Figure 1] Both physicochemical and biological profile are promising, as alkylamino substituted compounds combine nanomolar cytotoxic potencies with improved aqueous solubilities.
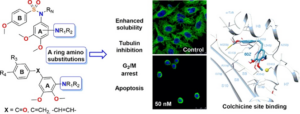
Figure. 1. Scheme of new amino analogs and experiments performed.
References
[1] E.C. McLoughlin, N.M. O’Boyle, Colchicine-Binding Site Inhibitors from Chemistry to Clinic: A Review, Pharmaceuticals 13 (1) (2020).
[2] M. Gonzalez, Y. Ellahioui, R. Alvarez, L. Gallego-Yerga, E. Caballero, A. Vicente-Blazquez, L. Ramudo, M. Marin, C. Sanz, M. Medarde, R. Pelaez, The Masked Polar Group Incorporation (MPGI) Strategy in Drug Design: Effects of Nitrogen Substitutions on Combretastatin and Isocombretastatin Tubulin Inhibitors, Molecules 24 (23) (2019).
Acknowledgements
Financial support came from Grant PID2021-127471OB-I00 funded by MCIN/AEI/ 10.13039/501100011033, Junta de Castilla y León and FEDER funds (SA0116P20) and USAL (PIC2-2022-01).



